Difference between revisions of "Conversion to UF"
(Created page with "<html><head></head><body> <div class="recommendation conversion_to_uf alert alert-success" role="alert" alt="Chemical conversion to an ultrafiltration membrane and then reuse....") |
|||
| (2 intermediate revisions by one user not shown) | |||
| Line 1: | Line 1: | ||
<html><head></head><body> | <html><head></head><body> | ||
| − | + | <div class="recommendation conversion_to_uf" style=""> | |
| − | <div class="recommendation conversion_to_uf" style=" | + | |
<table class="table table-bordered table-striped table-responsive"> | <table class="table table-bordered table-striped table-responsive"> | ||
<tbody><tr> | <tbody><tr> | ||
| Line 21: | Line 20: | ||
</table> | </table> | ||
</div> | </div> | ||
| − | <div class="recommendation conversion_to_uf" style=" | + | <div class="recommendation conversion_to_uf" style=""> |
</body></html> | </body></html> | ||
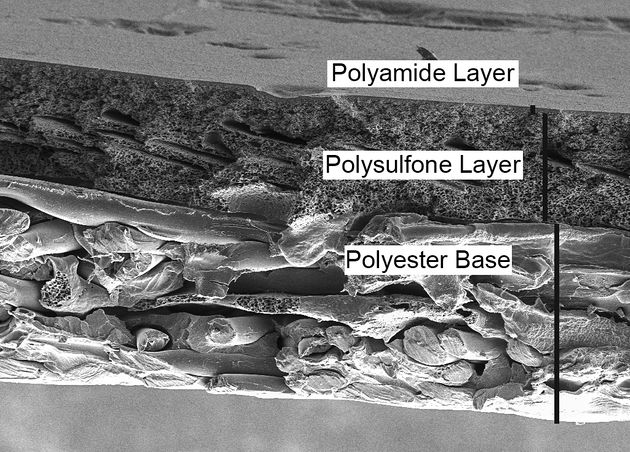
Given the nature of the composite construction used in RO production, relatively simple conversion from an RO to ultrafiltration (UF) membrane is possible<ref name="lawler-2014-F" />. The structure of an RO membrane can be seen in the image below, and flat sheet UF membranes have an extremely similar construction, but are just missing the top polyamide layer. By chemically degrading the polyamide active layer with sodium hypochlorite (NaClO), the polysulfone layer is exposed, resulting in a membrane with properties extremely similar to commercially available UF membranes<ref name="lawler-2014-G" />. | Given the nature of the composite construction used in RO production, relatively simple conversion from an RO to ultrafiltration (UF) membrane is possible<ref name="lawler-2014-F" />. The structure of an RO membrane can be seen in the image below, and flat sheet UF membranes have an extremely similar construction, but are just missing the top polyamide layer. By chemically degrading the polyamide active layer with sodium hypochlorite (NaClO), the polysulfone layer is exposed, resulting in a membrane with properties extremely similar to commercially available UF membranes<ref name="lawler-2014-G" />. | ||
| Line 37: | Line 36: | ||
<ref name="lawler-2014-G">Lawler, W.; Alvarez-Gaitan, J.; Leslie, G.; Le-Clech, P. "Assessment of End-of-Life Opportunities for Reverse Osmosis Membranes." The University of New South Wales (2015).</ref> | <ref name="lawler-2014-G">Lawler, W.; Alvarez-Gaitan, J.; Leslie, G.; Le-Clech, P. "Assessment of End-of-Life Opportunities for Reverse Osmosis Membranes." The University of New South Wales (2015).</ref> | ||
</references> | </references> | ||
| − | |||
Latest revision as of 22:08, 5 August 2015
| Compatible with plastic components | Yes |
| Compatible with fibreglass components | Yes |
| Available in Australia | Yes |
| Available globally | Yes |
| Manual disassembly required | No |
Cross section structure of an RO membrane.
The method for chemical conversion of RO membranes has been optimised, with controlled exposure to 300,000 ppm.hr of NaOCl resulting in organic and virus removal properties, and hydraulic performance, comparable to commercially available 10 – 30 kDa molecular weight cut off UF membranes. Potential applications for the converted RO membranes include use in pre-treatment filtration in desalination plant, waste water treatment or for low cost water treatment in developing areas.
This promising end-of-life application how now been extensively validated, and is ready for a trial application. If you require a series of extremely low cost, environmentally friendly, spiral wound UF membranes, please contact Pierre Le-Clech (p.le-clech@unsw.edu.au) for further information.